
The Meet&Learn by Tecnimede Group e-learning platform has been specifically designed to work alongside pharmacies as a tool providing support in professional training and keeping staff knowledge up to date.








Atlas Pharm
Located in Morocco, it was established in 2014 and commenced operations in 2015. It is focused on the production of highly active solid oral products within a containment environment for the treatment of oncological conditions, including breast, prostate, and blood cancer.
Atlas Pharm is equipped with the latest in pharmaceutical technology, with manufacturing processes supported by fully automated control systems.
It has sufficient production capacity to meet the manufacturing needs of the Tecnimede Group and our trading partners, based on the world’s best manufacturing practices.

Direction des Médicaments et de la Pharmacie – DMP (Moroccan Ministry of Health)
(National Authority of Medicines and Health Products of Portugal) I.P. – INFARMED
Current Good Manufacturing Practice (cGMP) - Infarmed
Agência Nacional de Vigilância Sanitária (Health Regulatory Agency of Brazil) – ANVISA

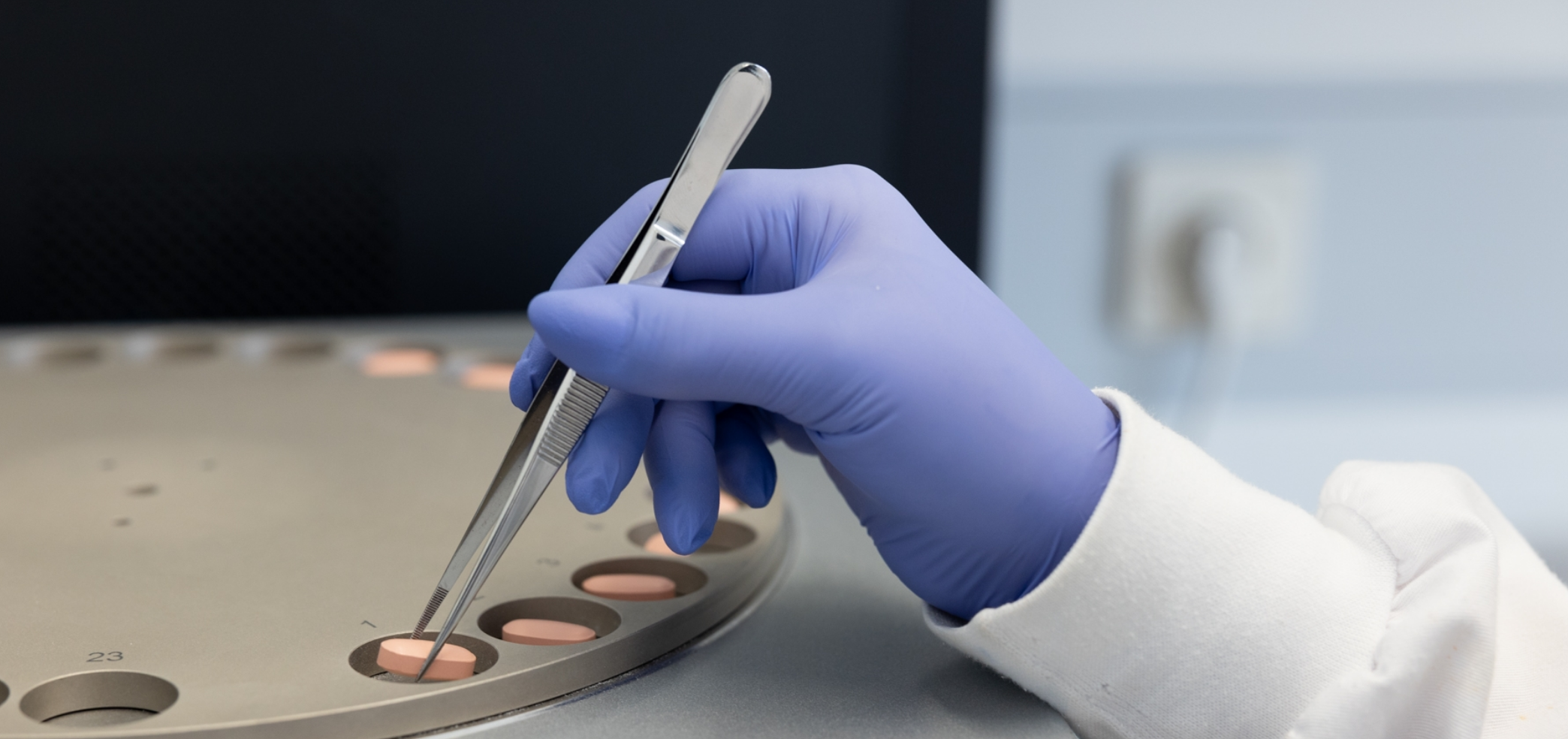
The investment in continuous improvement in terms of equipment, processes, and staff training, enables us to maintain high productivity levels and meet our customers’ high quality standards.

Quality Assurance and Control
We are equipped with the state-of-the-art tools necessary to carry out the different tests required by the different normative references.
Manufacturing Technology
Our manufacturing process is supported by electronic and automated control systems.
Quality Assurance and Control
We are equipped with the state-of-the-art tools necessary to carry out the different tests required by the different normative references.
Manufacturing Technology
Our manufacturing process is supported by electronic and automated control systems.

Tender & Hospital Business
This is the Tecnimede Group unit responsible for the marketing of all products within the hospital sector, with a special focus on the oncology and antiretroviral therapeutic segments.
The Tecnimede Group recognised the importance and specific nature of hospital management soon after its creation. For this reason, it created in 1995 its first business unit totally dedicated to the marketing of medicines for hospitals – Farmoz Hospitalar.
Currently, the Tecnimede Group, through its business area devoted to hospital tenders and negotiation—Tender & Hospital Business, closely monitors new challenges in hospital management, with a view to providing better accessibility to medicine, not only in Portugal, Spain, and Italy, but also in several other European countries, while enhancing dialogue between the various stakeholders.

Easier access to new generic medicines in the hospital environment to enable more patients to be treated
Treating more patients at a more affordable cost, favouring a better management of resources
Market sustainability, to avoid shortfalls and the loss of safe and affordable therapies
Better financing of hospitals to enable the best possible relationship between supply and procurement.

2nd April
World Autism Awareness Day
It is important to raise awareness of autism and promote and enhance its understanding by sharing information about the importance of early diagnosis and intervention.



2nd April
World Autism Awareness Day
It is important to raise awareness of autism and promote and enhance its understanding by sharing information about the importance of early diagnosis and intervention.

5th April
Portuguese National Rheumatoid Arthritis Patient Day
Rheumatoid arthritis affects around 40,000 patients in Portugal. It is considered to be the main systemic rheumatic disease due to its prevalence and the problems it causes. Its origin is unknown.



5th April
Portuguese National Rheumatoid Arthritis Patient Day
Rheumatoid arthritis affects around 40,000 patients in Portugal. It is considered to be the main systemic rheumatic disease due to its prevalence and the problems it causes. Its origin is unknown.

6th April
World Physical Activity Day
The World Health Organisation promotes this day, with the aim of promoting healthy and active lifestyles.



6th April
World Physical Activity Day
The World Health Organisation promotes this day, with the aim of promoting healthy and active lifestyles.
No
All hospital entities duly authorised for that purpose by INFARMED (National Authority of Medicines and Health Products of Portugal).
Over-the-Counter
Prescription-only medication.
What is meant by restricted POM – Point a)?
Medication subject to restricted medical prescription intended for exclusive hospital use on the grounds of its pharmacological properties, its innovative nature, or for public health reasons.
What is meant by restricted POM – Point b)?
Medication subject to restricted medical prescription intended for pathologies which can only be diagnosed in a hospital setting or in other specialised establishments with adequate diagnostic facilities, although patients may be prescribed medication and monitored outside these facilities.
What is meant by restricted POM – Point c)?
Medication intended for outpatient treatment, but its use can produce very serious side effects. As a result, it can only be prescribed by a specialist and requires special supervision for the duration of the treatment.
Find out here how to proceed in the event of issues relating to safety or to report a possible adverse event related to Tender&Hospital Business products.
Atlantic Pharma
Founded in 1984, Atlantic Pharma, located in Sintra in Portugal, is dedicated to the production of drugs for human use and its core mission is the Improvement and Preservation of Human Life and Health through the production of pharmaceutical products, with a strong focus on quality and technological innovation. Atlantic Pharma, with its capacity for the manufacturing of solids, semi-solids, liquids, and suspensions, guarantees not only the production needs of the Tecnimede Group but also those of our various partners.
The company's infrastructure was upgraded in 2000 to incorporate a modern production centre, with that mission statement in mind. That production centre meets the most stringent quality standards worldwide. Atlantic Pharma is equipped with the latest in technology and its production process is supported by electronic and automated control systems, which significantly reduce the risks associated with manual operations. A fully automated raw materials warehouse with a capacity of 6,000 pallets was installed in 2016. A new production unit was installed more recently, in 2019. It is equipped with the latest in pharmaceutical technology, aimed at producing scale-up batches and which will enable an increase in the production capacity of oral solid products.

Current Good Manufacturing Practice (cGMP) – Infarmed
(National Authority of Medicines and Health Products of Portugal) I.P. – INFARMED
Gulf Cooperation Council (GCC)
Jordan Food and Drug Administration (Jordan FDA)
Agência Nacional de Vigilância Sanitária (Health Regulatory Agency of Brazil) – ANVISA
Quality/ Envr./ Safety (NP EN ISO 9001:2015;NP EN ISO 14001:2015;NP ISO 45001:2019)
Manufacture of Investigational Medicinal Products
South Korean Authority
Russian Federation

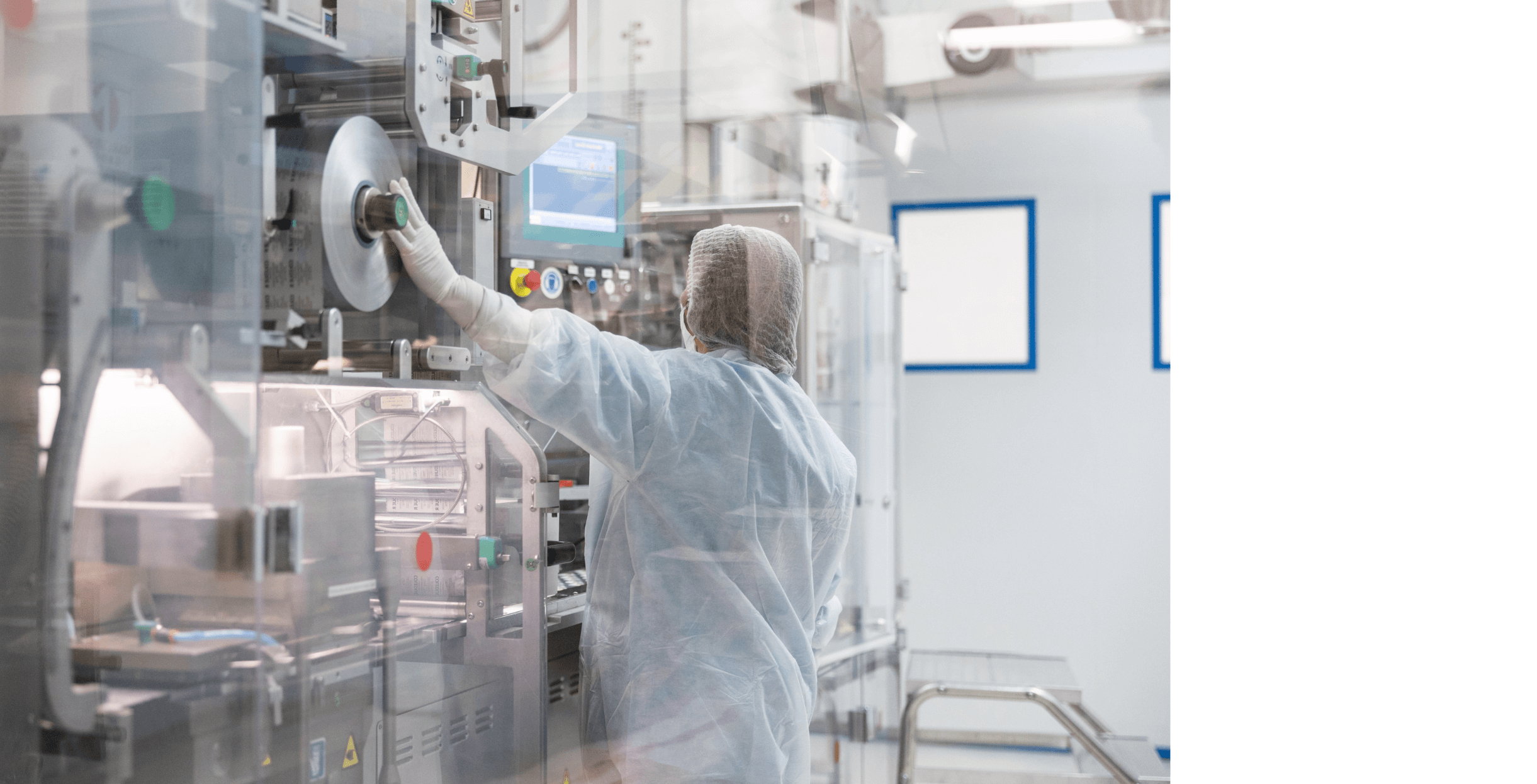
The investment in continuous improvement in terms of equipment, processes, and staff training, enables us to maintain high productivity levels and meet our customers’ high quality standards.

Quality Assurance and Control
We are equipped with the state-of-the-art tools necessary to carry out the different tests required by the different normative references.
Quality, Environment, and Safety Policy
Our Quality, Environment, and Safety Policy is governed by precise commitments.
Manufacturing Technology
Our manufacturing process is supported by electronic and automated control systems.
Quality Assurance and Control
We are equipped with the state-of-the-art tools necessary to carry out the different tests required by the different normative references.
Quality, Environment, and Safety Policy
Our Quality, Environment, and Safety Policy is governed by precise commitments.
Manufacturing Technology
Our manufacturing process is supported by electronic and automated control systems.

Tecnigen
Tecnigen is a manufacturer of generic pharmaceuticals, which is 100% Portuguese, yet global in scale. It resulted from the merger of Farmoz and Pentafarma.
Tecnigen, with operations in Portugal, Spain, and Italy, shares the same distinctive approach as the Tecnimede Group: it controls the entire cycle related to medications for human use through its strong commitment to Research & Development. Tecnigen has its own centre, Labor Qualitas, in Torres Vedras. Generic drugs are also produced in Portugal using state-of-the-art technology, at Atlantic Pharma, located in Sintra.
Tecnigen's diverse and comprehensive portfolio is divided into Prescription Medicines, Non-prescription Medicines, Dietary Supplements, and Cosmetics.

Tecnigen’s Vision
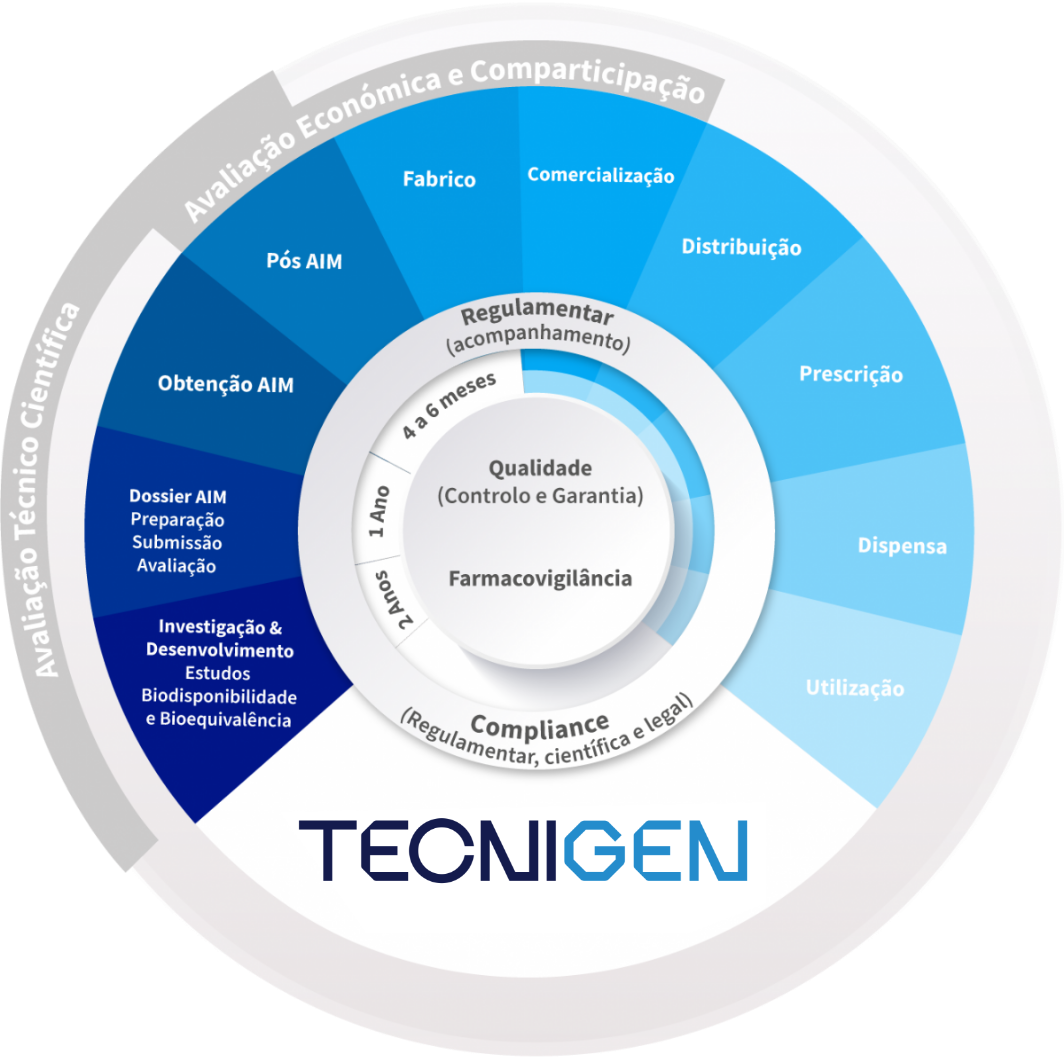
Tecnigen aims to establish and promote sustained partnerships with clients by creating adaptable and flexible business models. It also aims to consolidate and diversify the portfolio in relevant and innovative therapeutic areas and to continue to pioneer the launch of new generic products.
We now have, given the size and diversity of the portfolio, an even greater responsibility to patients, health professionals, and the Portuguese National Health Service, thus contributing to its long-term viability.
We believe that it is paramount in transactions to have brand Portugal present in all of our business activities, in order to generate value for the national economy.
3
In terms of turnover in the Ranking of Generic Drugs Laboratories
Top 5
And the only Portuguese company in the Ranking of Generic Drugs Laboratories
+650
References sold
75%
Of pharmacy needs covered by our portfolio


Tecnigen Events
Throughout the year, Tecnigen promotes various events that fit in with the strategy of creating value for partners and simultaneously supporting and enhancing the national economy.

Generic drugs: A new era
Tecnigen launch event, which included the conference “The New Frontiers of Health”, in partnership with Expresso newspaper.
Pharma Call: Meet, Learn & Act
Tecnigen promotes theoretical and practical training events in partnership with leading institutions, with the purpose of helping pharmacies to prepare themselves for current and future challenges.
Webinars
Analysis and discussion of study results based on population surveys, presentation of the 1st project carried out in Portugal involving a hospital and 30 community pharmacies in the distribution of medicines to outpatients, and an approach to the Science of Happiness were some of the featured topics in the webinars that have already taken place.
Generic drugs: A new era
Tecnigen launch event, which included the conference “The New Frontiers of Health”, in partnership with Expresso newspaper.
Pharma Call: Meet, Learn & Act
Tecnigen promotes theoretical and practical training events in partnership with leading institutions, with the purpose of helping pharmacies to prepare themselves for current and future challenges.
Webinars
Analysis and discussion of study results based on population surveys, presentation of the 1st project carried out in Portugal involving a hospital and 30 community pharmacies in the distribution of medicines to outpatients, and an approach to the Science of Happiness were some of the featured topics in the webinars that have already taken place.

A generic drug is a drug with the same qualitative and quantitative composition in terms of active substances and the same pharmaceutical form as the reference drug. The bioequivalence of generic drugs in relation to the reference drug is also guaranteed through appropriate bioavailability studies.
Two pharmaceutical products are bioequivalent if they are pharmacokinetically equivalent, i.e. if they have similar levels of bioavailability (rate and extent of absorption) after administration at the same molar dose. Therefore, the efficacy and safety profile are expected to be essentially the same. Pharmaceutical equivalence implies the same quantity of the same active substance, in the same pharmaceutical form and dosage, via the same route of administration, and meeting the same or comparable standards.*
The Tecnimede Group promotes the performance of bioequivalence studies that qualify generic drugs for both domestic and international markets.
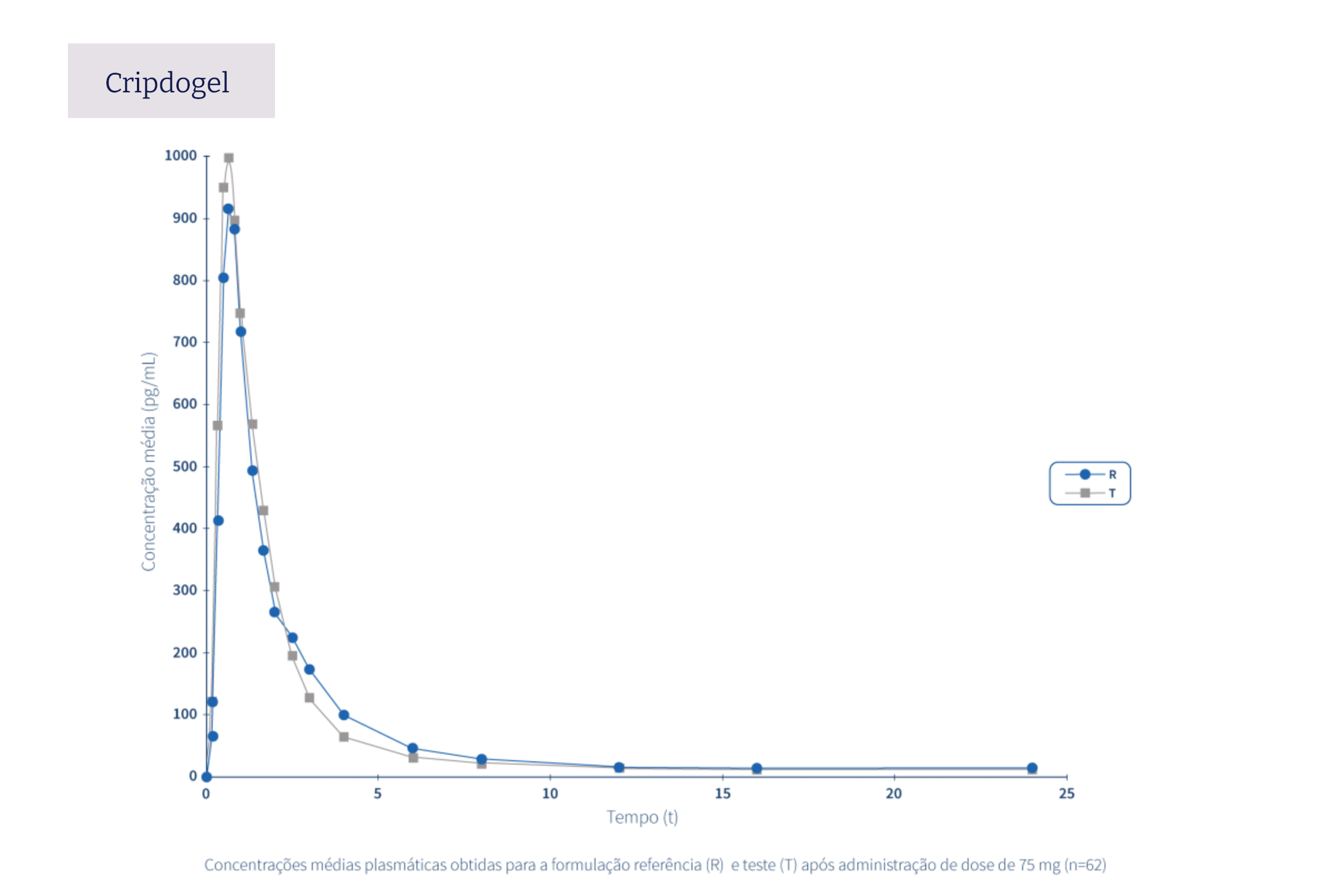
The following graphs exemplify the intense clinical research and integrated project management work that allows the Tecnimede Group to provide essential medicines for the treatment and prevention of the most varied illnesses and clinical conditions.
*Birkett DJ. Generics - equal or not?. Aust Prescr 2003;26:85-7.

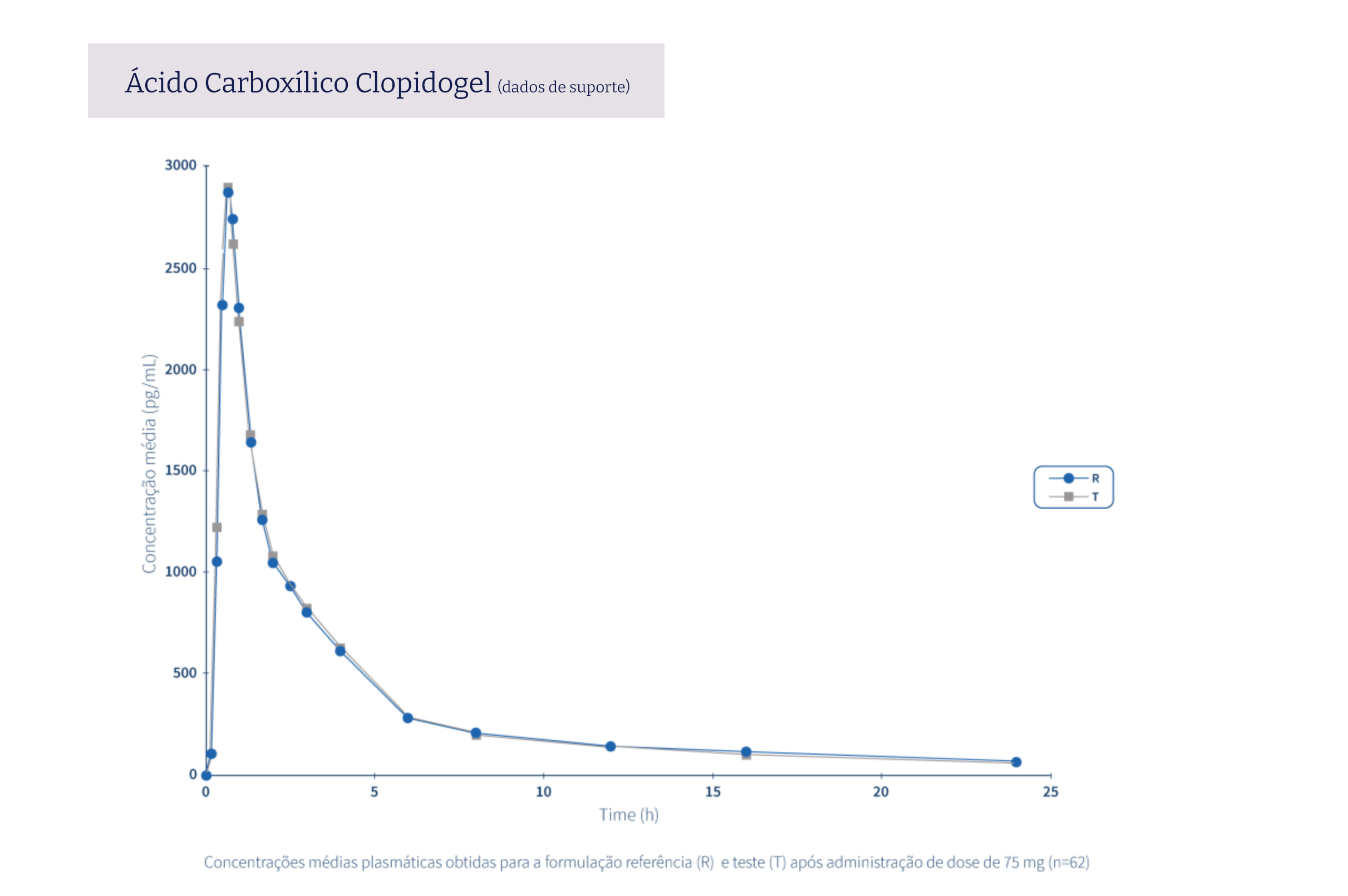

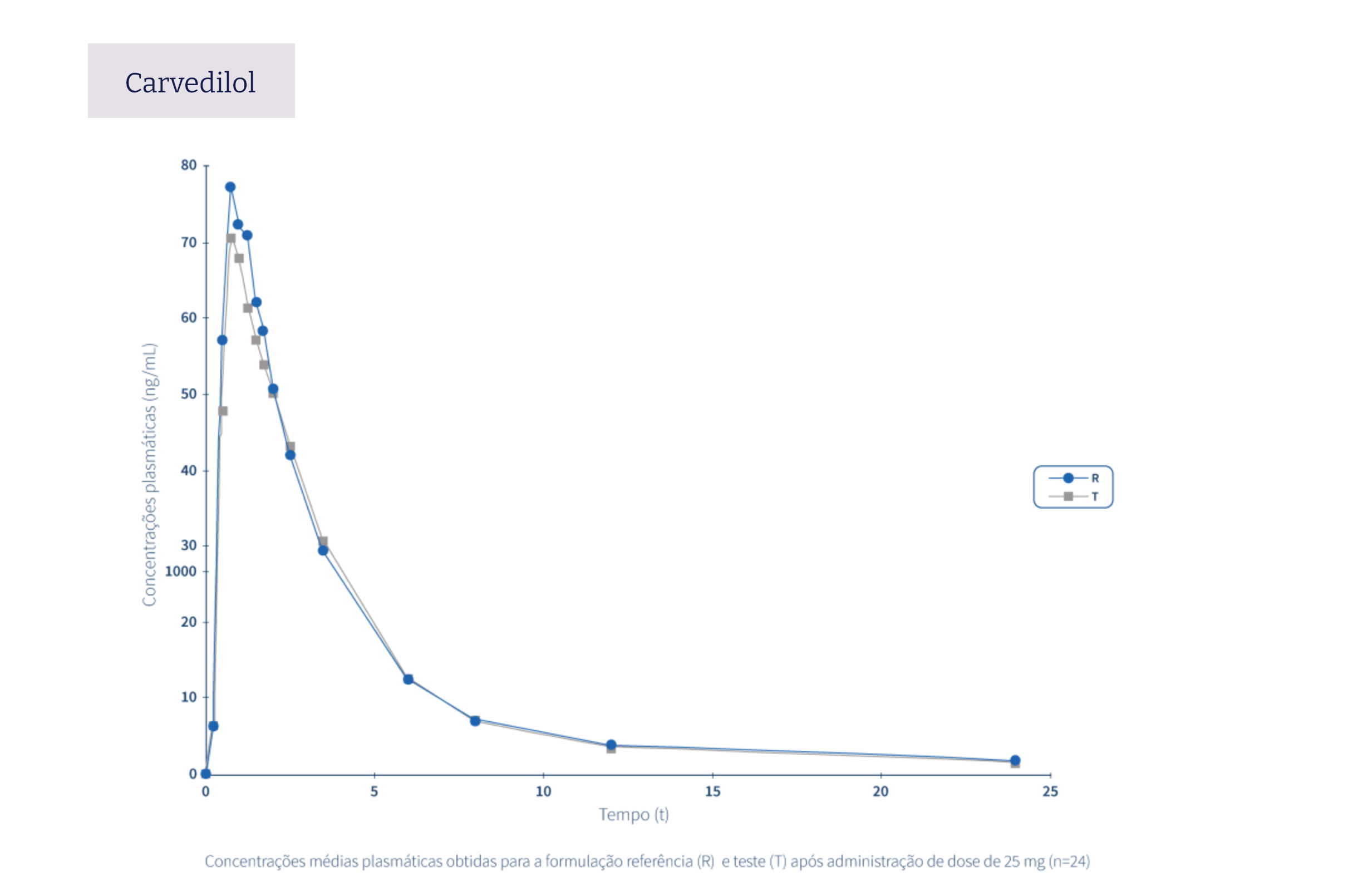

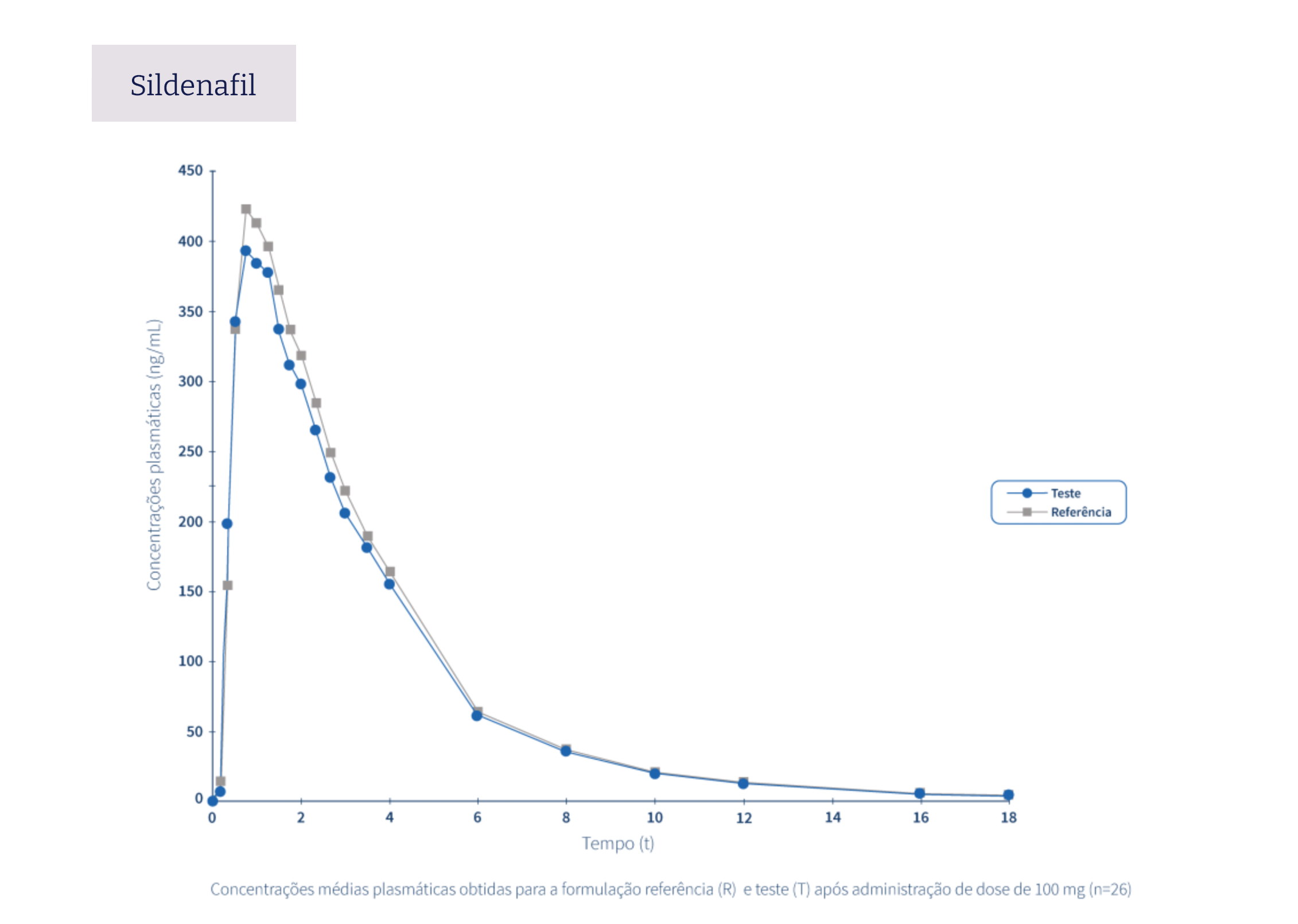

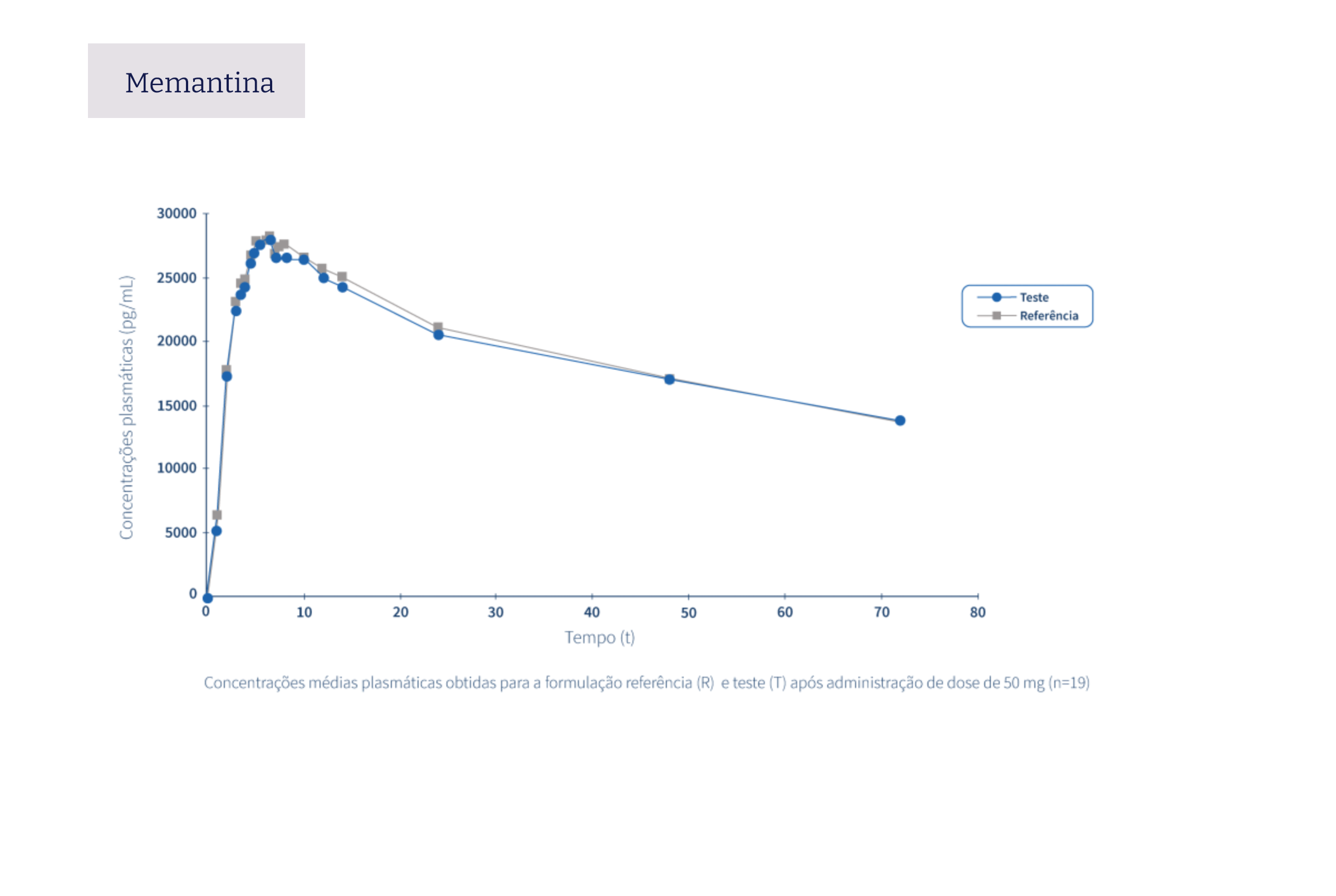

Pharmacovigilance encompasses all safety monitoring activities concerning medicines granted marketing authorisation, through the collection and assessment of adverse drug reaction (ADR) reports received by the National Pharmacovigilance System (SNF), the identification of risks associated with the use of medicines, their assessment, the implementation of risk minimisation measures, and their communication to health professionals, patients, consumers, and the general public. Find out here how to proceed in the event of issues relating to safety or to report a possible adverse event related to Tecnigen products.

The Meet&Learn by Tecnimede Group e-learning platform has been specifically designed to work alongside pharmacies as a tool providing support in professional training and keeping staff knowledge up to date.
MedOn.pt is a 100% Portuguese online store, whose main objective is the sale of non-prescription medicines and other health products, including Tecnigen products.
Tecnimede
Tecnimede, founded in 1980, was the Group's first company and the one that gave it its name.
Ever since its inception, Tecnimede has always been defined by its dynamic approach and has been recognised as a partner by all healthcare professionals.
With a focus on new products, the company's offering includes a portfolio of prescription medications and innovative products, thereby contributing to the well-being of the community and the improvement of the quality of life of the general public.
Tecnimede is also known for the support it provides with regard to scientific training. The Tecnishare platform is an example of this, which is aimed at healthcare professionals, providing them with access to varied useful content to assist them in their day-to-day work.



Main Therapeutic Areas
Tecnimede has made its mark in the main therapeutic areas, through a broad portfolio of prescription medications and innovative products.

Cardiometabolic Health
According to the Organisation for Economic Co-operation and Development (OECD), cardiovascular diseases, such as Hypertension or Diabetes, remain the primary causes of death in OECD countries.
Women's and Men's Health
Women's and Men's Health are two of Tecnimede's core therapeutic areas, with a portfolio directed to diseases such as Genitourinary Syndrome of Menopause, Urinary Incontinence, or Benign Prostate Hyperplasia.
Musculoskeletal Disorders
Vitamin D deficiency and Osteoporosis are two conditions which are very prevalent in Portugal and which have a negative impact on the health of the population.
Pain
According to the International Association for the Study of Pain (IASP) pain is an unpleasant sensory and emotional experience associated with or akin to that associated with actual or potential tissue damage. In 2012, 37% of the adult population in Portugal suffered from chronic pain.
Cardiometabolic Health
According to the Organisation for Economic Co-operation and Development (OECD), cardiovascular diseases, such as Hypertension or Diabetes, remain the primary causes of death in OECD countries.
Women's and Men's Health
Women's and Men's Health are two of Tecnimede's core therapeutic areas, with a portfolio directed to diseases such as Genitourinary Syndrome of Menopause, Urinary Incontinence, or Benign Prostate Hyperplasia.
Musculoskeletal Disorders
Vitamin D deficiency and Osteoporosis are two conditions which are very prevalent in Portugal and which have a negative impact on the health of the population.
Pain
According to the International Association for the Study of Pain (IASP) pain is an unpleasant sensory and emotional experience associated with or akin to that associated with actual or potential tissue damage. In 2012, 37% of the adult population in Portugal suffered from chronic pain.

Find out here how to proceed in the event of issues relating to safety or to report a possible adverse event related to Tecnimede products.

Time to get Stronger
Tempo de Fortalecer is a unique project developed by Tecnimede for medical professionals and the general public that sheds new light on one of the most natural phases of a woman's life cycle: menopause
Even though menopause is a natural part of a woman's life cycle, this phase has a significant impact on life in various different ways, affecting both family and professional life.
The main objective of this platform is to address menopause and the pathologies that affect women during this phase.

The Tecnishare platform is aimed at healthcare professionals providing them with access to varied and useful content for their day-to-day clinical practice. This digital platform enables healthcare professionals to improve their scientific knowledge by providing a range of content designed to support clinical practice in several of Tecnimede's core therapeutic areas: cardiometabolic health, musculoskeletal disorders, pain, women's health, men's health, and the central nervous system. For example, with regard to the available content, Tecnishare provides therapeutic treatment algorithms, equianalgesia calculators, training videos, along with many other things. There is an area in Tecnishare reserved for the Tecnimede team which provides access to learning and training resources, thus offering better access to contents supporting the medical profession.
Tecnimede Consumer Healthcare
Tecnimede Consumer Healthcare emerged in 2016 as a natural response to the dynamics of the market in Portugal. It aims to bring new and better solutions for the pharmaceutical market in order to promote the well-being of consumers, day after day.
This business unit is dedicated to the marketing of non-prescription products, such as over-the-counter (OTC) medications, medical devices, dietary supplements, and cosmetics.
The Tecnimede Group has invested and continues to invest in this new business unit because it not only wants to provide new and improved solutions for the pharmaceutical industry, but also to help meet the needs of consumers and to promote their well-being day after day.

Our Areas
of Expertise
Some of the Tecnimede Group’s best-known brands, such as Broncoliber, Magnesiocard, and the oral health brand, Curaprox, excel in the following market areas.






Find out here how to proceed in the event of issues relating to safety or to report a possible adverse event related to Tecnimede Consumer Healthcare products.

The Meet&Learn by Tecnimede Group e-learning platform has been specifically designed to work alongside pharmacies as a tool providing support in professional training and keeping staff knowledge up to date.
MedOn.pt is a 100% Portuguese online store, whose main objective is the sale of non-prescription medicines and other health products, including Tecnigen products.
Tecnimede Italy
Tecnigen Italy, a subsidiary of the Tecnimede Group, began trading in the Italian pharmaceutical industry in 2014.
With a focus on generic drugs, Tecnigen Italia covers the main therapeutic areas of the generic pharmaceutical market. Most of the products are the result of its own research, development, and production, thanks to the Research and Development centre, Labor Qualitas, and the Atlantic Pharma production centre, which is dedicated to the production of medicines for human use.
Tecnigen’s presence in Italy has been going from strength to strength, first with the launch of a range of over-the-counter medicines and then with the launch of a range of dietary supplements.
Tecnigen Italy aims to continue to invest in healthcare through new developments and diversification within the Italian pharmaceutical industry.
Tecnimede Spain
Tecnimede Spain , the Spanish subsidiary of the Tecnimede Group, began trading in the Spanish pharmaceutical industry in 2009 with Tecnigen, the Group’s brand focused on generic drugs.
From the very beginning, Tecnimede Spain has always maintained a policy of progressive expansion, in which each growth step is the result of prior work to consolidate the results that have already been achieved. Today, Tecnimede Spain boasts a business network that spans across the whole of Spain and facilitates the marketing of more than 84 different molecules, generating a total of more than 180 generic drug references.
Tecnimede Spain covers the most important therapeutic fields of the generic pharmaceutical industry, and most of its products are the result of its own research, development, and manufacturing, thanks to the Research and Development centre, Labor Qualitas, and the Atlantic Pharma production centre.
Since 2019, Tecnimede Spain has been going from strength to strength in the Spanish pharmaceutical sector, first with the launch of a line of over-the-counter medicines and then later with the launch of a range of dietary supplements.
Tecnimede Spain will continue to invest in healthcare through new developments and diversification within the Spanish pharmaceutical industry.

Altadis
Altadis was created in 2015 as the result of a joint venture between the Tecnimede Group and the Colombian company Altea Farmacêutica.
Altadis is committed to working at the forefront of the latest advances in healthcare technology and pharmaceuticals related to the central nervous system, cardiology, oncology, immunomodulators, and biotechnology.
Altadis has recently expanded its sales team to provide better coverage across Portugal. This achievement is aligned with Altadis’ goal of becoming recognised as one of the top five best laboratories within the relevant markets by marketing at least five different therapeutic product ranges.
Altadis hopes to continue to expand in the Colombian pharmaceutical market in the coming years, through the development of new molecules (designed and manufactured within the Tecnimede Group), as well as through investment in new areas, such as primary care.
Altadis is presently formed of a team of committed professionals with a strong sense of belonging. It is a professional team constantly looking for ways to improve, driven to successfully meet the needs of pharmacists, doctors, researchers, and society as a whole.
