Tecnigen is a manufacturer of generic pharmaceuticals, which is 100% Portuguese, yet global in scale. It resulted from the merger of Farmoz and Pentafarma.
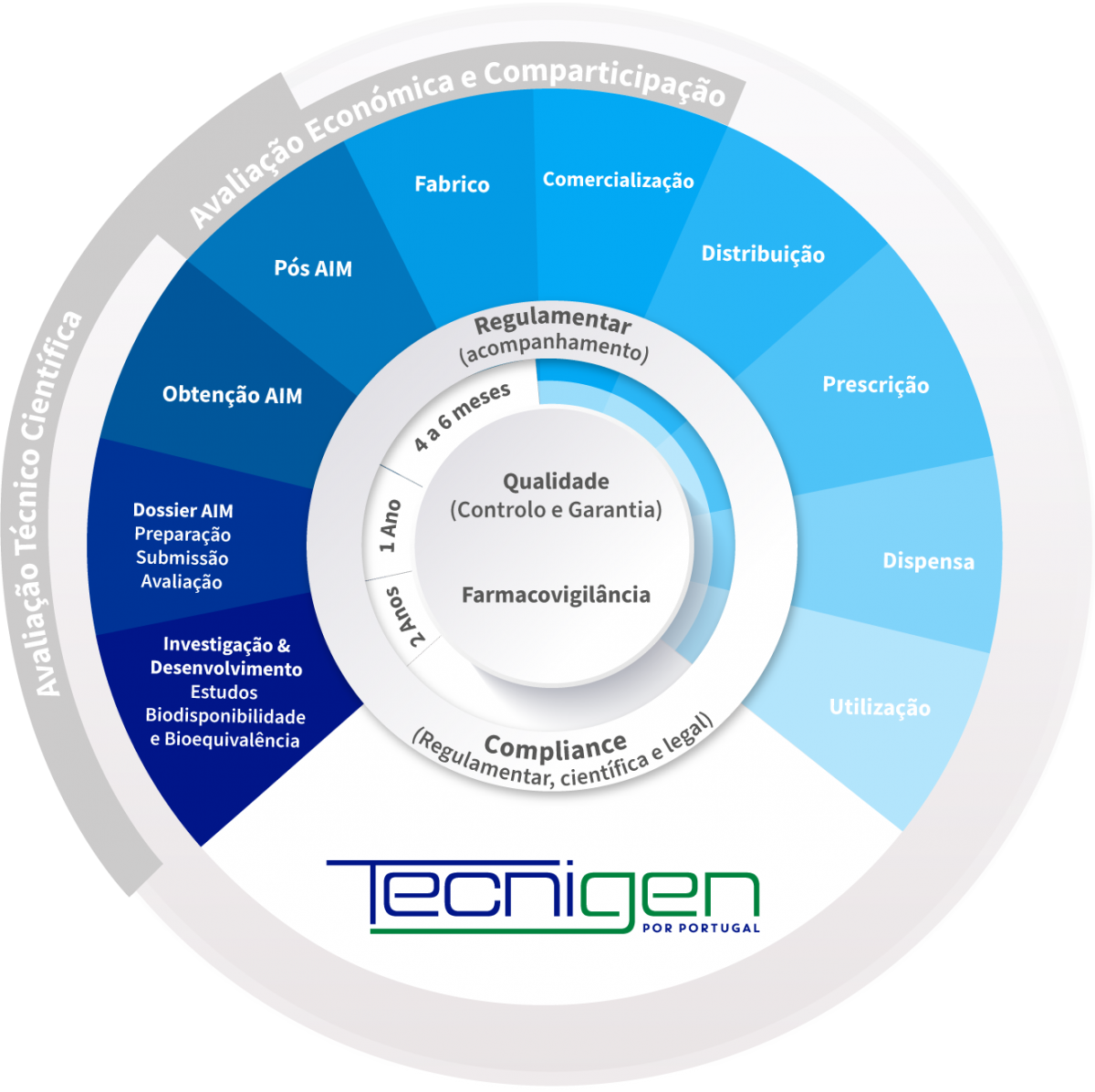
Tecnigen, with operations in Portugal, Spain, and Italy, shares the same distinctive approach as the Tecnimede Group: it controls the entire cycle related to medications for human use through its strong commitment to Research & Development. Tecnigen has its own centre, Labor Qualitas, in Torres Vedras. Generic drugs are also produced in Portugal using state-of-the-art technology, at Atlantic Pharma, located in Sintra.
Tecnigen's diverse and comprehensive portfolio is divided into Prescription Medicines, Non-prescription Medicines, Dietary Supplements, and Cosmetics.
Tecnigen aims to establish and promote sustained partnerships with clients by creating adaptable and flexible business models. It also aims to consolidate and diversify the portfolio in relevant and innovative therapeutic areas and to continue to pioneer the launch of new generic products.
We now have, given the size and diversity of the portfolio, an even greater responsibility to patients, health professionals, and the Portuguese National Health Service, thus contributing to its long-term viability.
We believe that it is paramount in transactions to have brand Portugal present in all of our business activities, in order to generate value for the national economy.
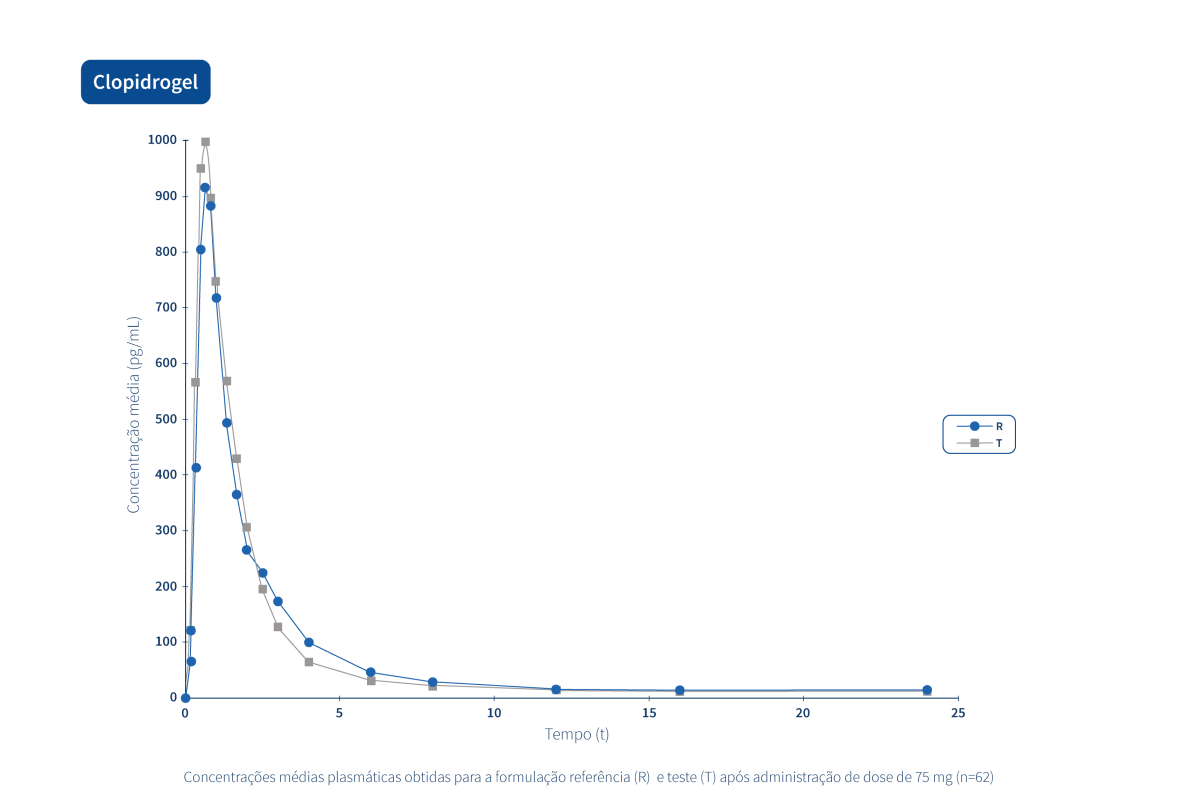
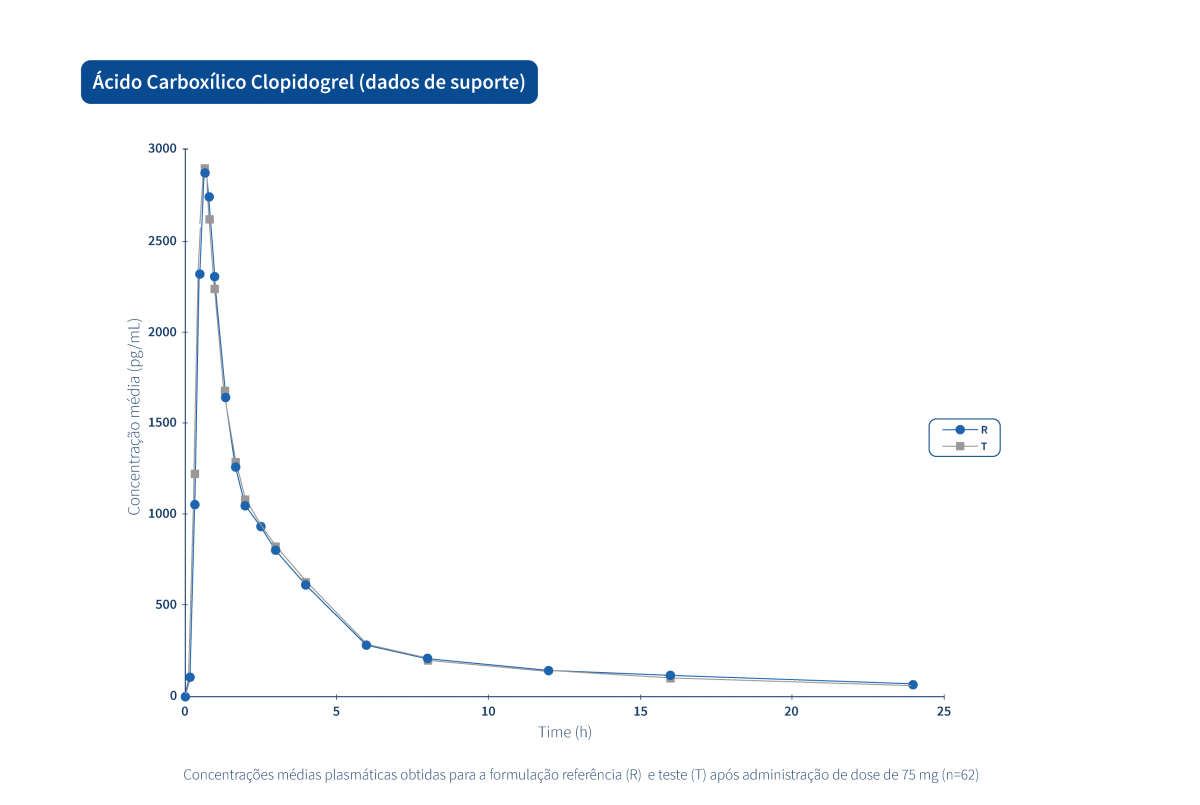
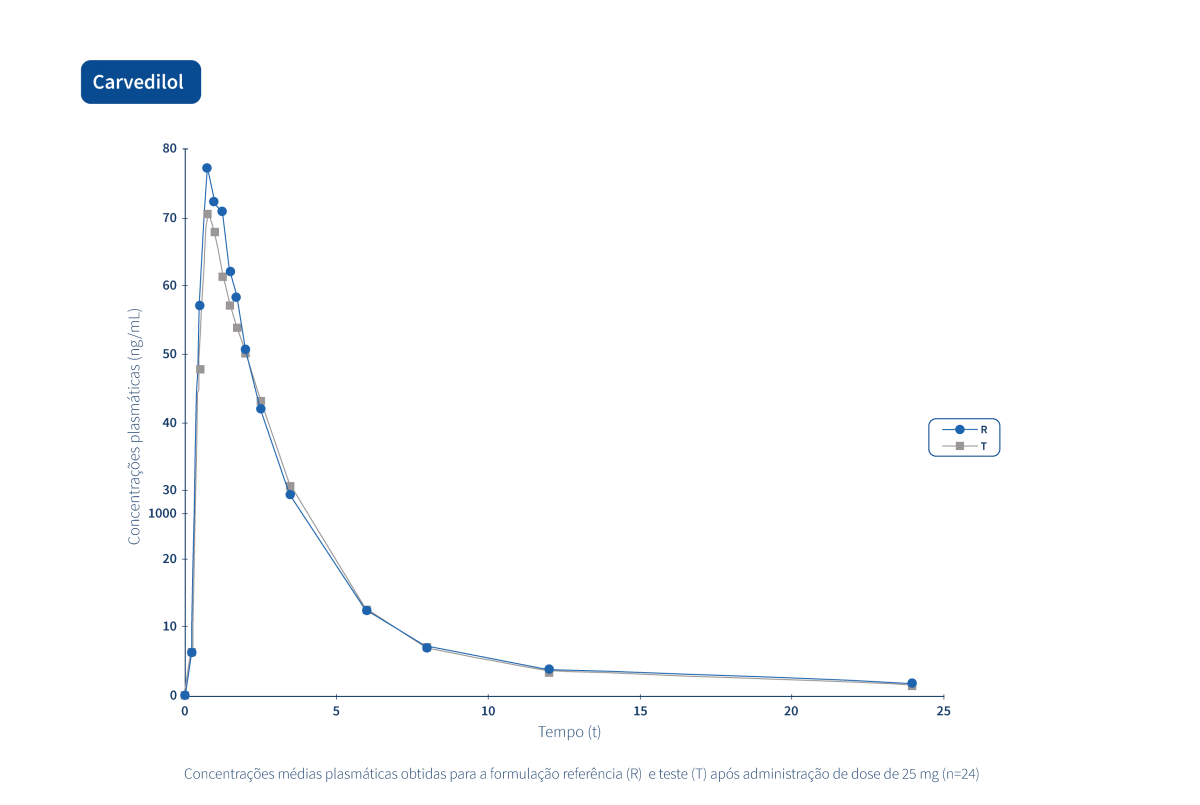
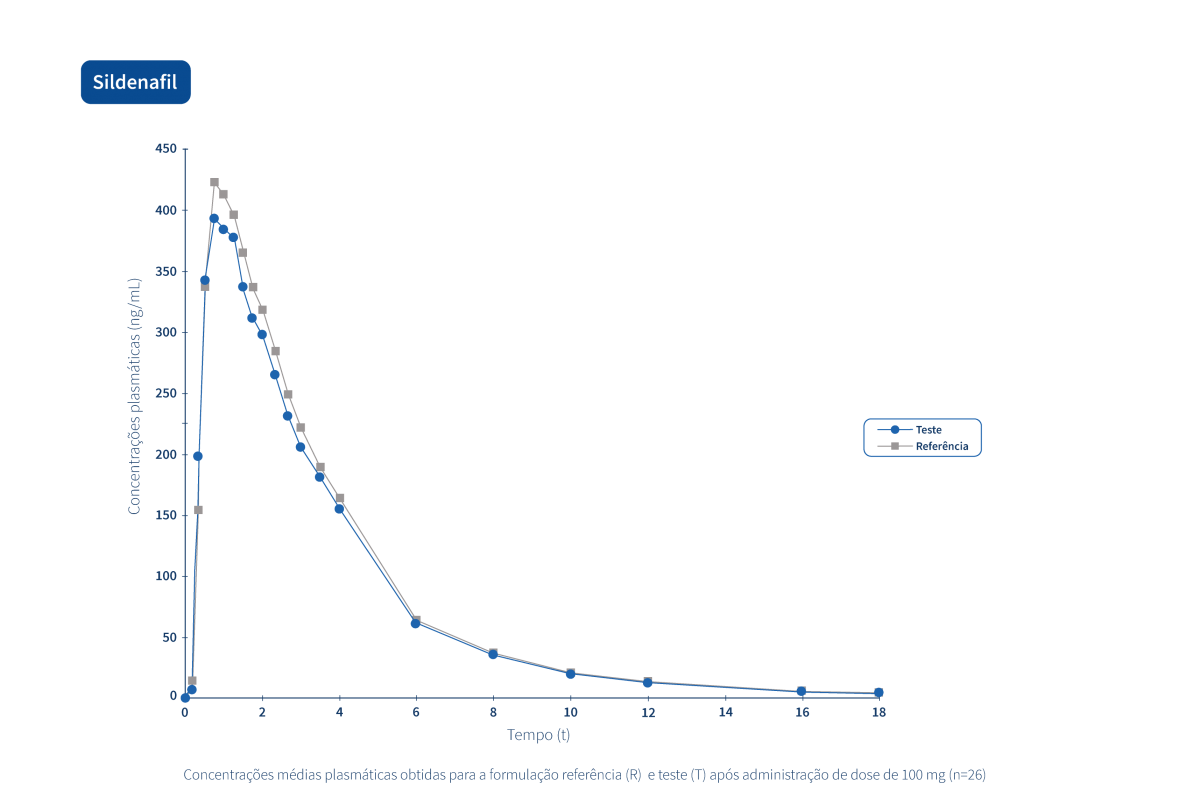
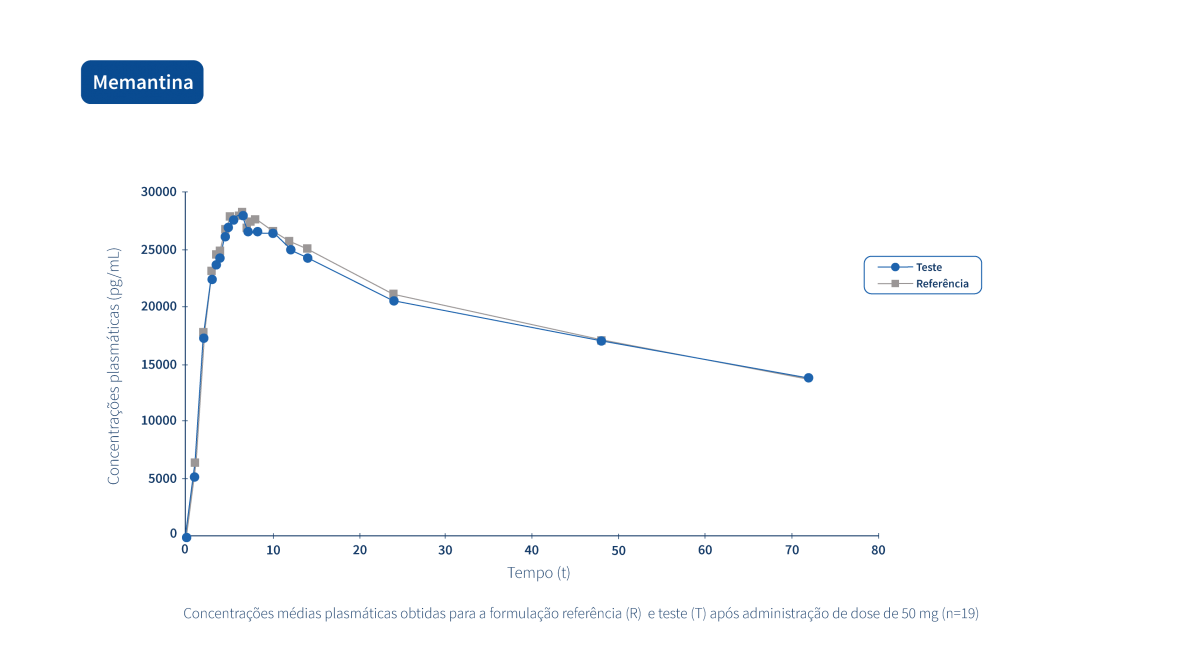
A generic drug is a drug with the same qualitative and quantitative composition in terms of active substances and the same pharmaceutical form as the reference drug. The bioequivalence of generic drugs in relation to the reference drug is also guaranteed through appropriate bioavailability studies.
Two pharmaceutical products are bioequivalent if they are pharmacokinetically equivalent, i.e. if they have similar levels of bioavailability (rate and extent of absorption) after administration at the same molar dose. Therefore, the efficacy and safety profile are expected to be essentially the same. Pharmaceutical equivalence implies the same quantity of the same active substance, in the same pharmaceutical form and dosage, via the same route of administration, and meeting the same or comparable standards.*
The Tecnimede Group promotes the performance of bioequivalence studies that qualify generic drugs for both domestic and international markets.
The following graphs exemplify the intense clinical research and integrated project management work that allows the Tecnimede Group to provide essential medicines for the treatment and prevention of the most varied illnesses and clinical conditions.
*Birkett DJ. Generics - equal or not?. Aust Prescr 2003;26:85-7.
Pharmacovigilance encompasses all safety monitoring activities concerning medicines granted marketing authorisation, through the collection and assessment of adverse drug reaction (ADR) reports received by the National Pharmacovigilance System (SNF), the identification of risks associated with the use of medicines, their assessment, the implementation of risk minimisation measures, and their communication to health professionals, patients, consumers, and the general public. Find out here how to proceed in the event of issues relating to safety or to report a possible adverse event related to Tecnigen products.